Assistant Professor Dr. Shatha Abd Alameer Jawad
And: Salwa Jassim Haji
Department of chemistry, College of Education for Pure Sciences.
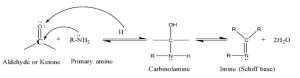
Schiff bases are organic compounds that contain a double bond between carbon and nitrogen (C=N), which is called azomethine. The azomethine group has become competitive with the carbonyl group (CO) as a working group in organic chemistry, and in some cases it is superior to it. In terms of their ability to form complexes, many imines have liquid crystalline properties [1]. Schiff bases were prepared for the first time in 1864 by the German scientist Hugo Schiff By the process of condensation of aldehydes or ketones with aromatic or aliphatic primary amines [2]. Schiff base ligands are a very important class in coordination chemistry because they have many applications in various fields in recent years, Interest in preparing and studying the properties of transition element complexes containing ligands of Schiff bases has increased significantly, as they are considered to be of great importance in many biological reactions. , especially in the process of nutritional catabolism of amino acids and carbonyl compounds that consist of proteins and carbohydrates, respectively [3]. Schiff bases are similar to aldehyde or ketone compounds, but the carbonyl group is replaced by an imine or azomethine group. Schiff bases containing aryl substituents are more stable and can be formed more quickly than those containing alkyl substituents. The properties and stability of Schiff bases depend mainly on the carbonyl compounds as well as on the type of amines, whether aliphatic or aromatic. Schiff bases prepared from aromatic amine and aromatic aldehyde are the most stable among Schiff bases, and this is due to increased resonance stability. In addition, the stability of these types of compounds also depends on the amine used, the aldehydes and ketones. Schiff bases that consist of aliphatic aldehydes are unstable and ready to polymerize, while aromatic aldehydes that have effective electron exchange are more stable. The reaction to form a Schiff base from aldehydes or ketones is a reversible reaction and usually occurs in the presence of an acidic or basic catalyst or in the presence of heating [4] as in the following diagram:
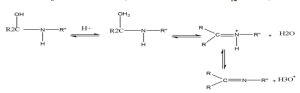
The above condensation reaction is a reversible reaction because due to the presence of a water molecule formed as a result of the condensation reaction, the products can dissociate and give the reactants themselves. . This is called Schiff base hydrolysis. It has been observed that Schiff bases produced by aphatic amines undergo slow hydrolysis because they form an intermediate ring of implicit hydrogen bonding[5]. The formation of these compounds is governed by the mechanism of nucleophilic addition to the carbonyl group. In this case the nucleophile is an amine, In the first part of this mechanism, the amine reacts with the aldehyde or ketone to obtain an unstable additive compound called (Carbinolamine) which loses a water molecule by an acidic or basic catalyst, as in the following diagram.
Of course, the process of withdrawing a water molecule from the intermediate compound (Carbinolamine) is the step that determines the speed of this reaction. . It also gives the reason for stimulating this reaction with acids, and the acid must be unconcentrated because the basic amine in this case will react with it and become nucleophilic, thus losing its nucleophilic character [6]. Therefore, the equilibrium is shifted to the left of the reaction, and thus the intermediate compound (Carbinolamine) will not be formed. Therefore, most reactions for preparing Schiff bases occur in a medium with moderate acidity. Imines and their metal complexes are a class of compounds that have been widely studied due to their chemical activity, chelating ability, physical properties, and multiple applications in various fields, including industrial fields. They were used as corrosion inhibitors, catalysts, and in the preparation of polymers. Imines were also used in polymers to improve electrical conductivity properties. Imine complexes have also been used to prepare inks and dyes[6], as well as for biological industrial purposes, where they have shown antimalarial, antifungal, antiviral, and antibacterial effects. These compounds with transition metals form colorful chelating complexes used for accurate qualitative and quantitative identification of many metal ions. Imines are also used in diagnosis, quantitative analysis, separation of compounds containing a carbonyl group, and other important applications of Schiff bases[7].
References
[1] Y. Xin and J. Yuan, “Schiff’s base as a stimuli-responsive linker in polymer chemistry,” Polymer Chemistry, vol. 3, no. 11, pp. 3045-3055, 2012.
[2] E. Yousif, A. Majeed, K. Al-Sammarrae, N. Salih, J. Salimon, and B. Abdullah, “Metal complexes of Schiff base: preparation, characterization and antibacterial activity,” Arabian Journal of Chemistry, vol. 10, pp. S1639-S1644, 2017.
[3] M. A. Ashraf, K. Mahmood, A. Wajid, M. J. Maah, and I. Yusoff, “Synthesis, characterization and biological activity of Schiff bases,” IPCBEE, vol. 10, no. 1, p. 185, 2011.
[4] Y. Wang et al., “Evaluation of schiff-base covalent organic frameworks for CO2 capture: structure–performance relationships, stability, and performance under wet conditions,” ACS Sustainable Chemistry & Engineering, vol. 10, no. 1, pp. 332-341, 2021.
[5] T. Qin et al., “Recent progress in conductive self‐healing hydrogels for flexible sensors,” Journal of Polymer Science, vol. 60, no. 18, pp. 2607-2634, 2022.
[6] D. S. Timofeeva, A. R. Ofial, and H. Mayr, “Nucleophilic reactivities of Schiff base derivatives of amino acids,” Tetrahedron, vol. 75, no. 4, pp. 459-463, 2019.
[7] D. S. Schiff, “Looking through a policy window with tinted glasses: Setting the agenda for US AI policy,” Review of Policy Research, vol. 40, no. 5, pp. 729-756, 2023.