Prof.Asst. Dr. Shatha abd-ala meer Jawad
Dhuha Mahdi AL-Baharani
College name: College of Education for Pure Sciences / University of karbala
Amides compound are even or open binary amides compound, and they are in the following
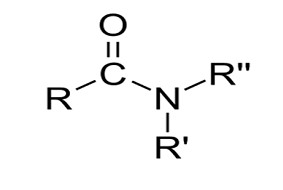
An amide in organic chemistry [1][2] The compound RC(=O)NR′R′′, where R, R’, and R′′, is an organic molecule. stand in for each group.Usually, hydrogen atoms or organyl substances with the formula [3], also known as a carboxamide or an organic amide.[4][5] When an amino acid side chain has an amide group, such the amino acid asparagine, and glutamineit’s referred to as an isopeptide bond.
Peptide bonds are what occur when the amide group is located in the protein’s primary chain. It can be considered a derivative of a carboxylic acid (RC(=O)OH). the amine group being (NR′R) has assumed place of the hydroxyl group (OH), or alternatively as a link between an acyl. An amine group with a group (alkanoyl) (RC(=O)). Typical amides include dimethylformamide (H(=O)N(CH3)2), acetamide (H3C(=O)NH2), benzamide (C6H5(=O)NH2), and formamide (H(=O)NH2).. Chloroformamide (Cl(=O)NH2) and N-chloroacetamide (H3C(=O)NHCl) are two unusual instances of amides. According on Amines are categorized depending on Whether the amine subgroup is NH2, NHR, or NRR, where R and R’ are primary, secondary, and tertiary groups other than hydrogen1. [In body: untrustworthy]. The amide group, specifically, the The central The carboxamide group is found in amides as C(=O)(N). In both nature and technology, amides are everywhere. Important polymers with amide group connections include common polymer compounds including Nylons, Aramid, Twaron, and Kevlar, as well as proteins. These linkages are simple to produce, provide structural stability and hydrolysis resistance. Along with several further noteworthy biological substances, amides also include several medications including LSD, Penicillin as well as paracetamol.[6]. Common solvents include low-molecular-weight amides like dimethylformamide
The word “amide” is added after the name of the parent acid in the nomenclature that is considered to be conventional. For instancethe amide created from acetic acid is known as acetamide (CH3CONH2). IUPAC recommends using ethanamide as the official word., But it is rarely employed.. The nitrogen-containing substituents are listed at the top of the nomenclature. N,N-dimethylacetamide, or CH3CONMe2 (Me = CH3), is the amide produced when dimethylamine and acetic acid react.
Dimethylacetamide is a frequent name for this chemical as well. It is true that lactams are secondary or tertiary amides, also known as cyclic amides [7].
Applications
Amides are widely present in both the natural and man-made worlds. The bulk of biological macromolecules are composed of peptides joined by amide bonds; some human-made polymers also employ this technique.
Properties
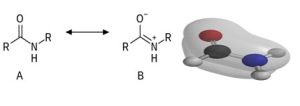
Bonding: Molecular orbitals for the formamide molecule are depicted in gray in a ball-and-stick model. A portion The nitrogen atom’s only pair of electrons combine with the two electrons on the carbon atom to form a double bond. the carbonyl group should delocalize. Because delocalized electrons are present in their The O, C, and N atoms really form a conjugated system as shown by their molecular orbitals. The outcome is unlike amines, which have three pyramidal nitrogen bonds, amides have three planar nitrogen bonds. This planar limitation prevents rotations around the N bond, which has a substantial impact on the bulk mechanical properties of such molecules… A resonance between the neutral (A) and zwitterionic (B) alternative structures may also be used to explain the structure of an amide [8].
Basicity
Amides are much weaker bases than amines compound. An amide’s conjugate acid has a pKa of around 0.5, whereas an amine’s has a pKa that’s approximately a value of 9 As a result, amides’ of around 0.5.. As a result, amides’ acid-base characteristics in water are less obvious. The carbonyl’s removal of electrons from the amine explains the minimal basicness in comparison. Contrarily, the conjugate acids of amides are far more potent bases with pKas between 6 and 10 which are more powerful than Aldehydes, oxy carboxylic acids, esters, and ketones ] 9 [.
Hydrogen bonding and solubility
The carbonyl (C=O), which has a greater dipole than the N-C dipole, has a higher electronegativity. Because they have a C=O dipole and, to a lesser extent, an N-C dipole, amino acids can act as H-bond acceptors. Amides can act as H-bond donors in primary and secondary amides thanks to N-H dipoles. As a result, additional chemicals such as amides may produce hydrogen bonds.Amidates and esters have approximately the same solubilities. Compared to comparable amides, amines and carboxylic acids are usually more soluble because they may both contribute to and absorb hydrogen bonds. With the notable exception of N,N-dimethylformamide, tertiary amides have a poor water solubility.both protic solvents and water
Reactions
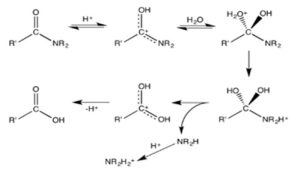
Mechanism of an amide’s acid hydrolysis [9]. Despite being less reactive than esters, amide compounds, they nonetheless undergo several chemical reactions. Both hot, alkaline and extremely acidic situations cause amides to hydrolyze. Under alkaline circumstances, basic hydrolysis does not result in the formation of carboxylate ion and ammonia, but rather carboxylic acid and the ammonium ion. These reactions are non-catalytic and irreversible due to the initial amine’s protonation in an acidic environment and the initial carboxylic acid’s deprotonation in a basic environment. Amides are adaptable building blocks for a variety of different functional divisions. The carbonyl oxygen reacts with electrophilesUsually, this happens prior to hydrolysis, which is facilitated by both Lewis and Brnsted acids…. It is known that enzymes, such as peptidases and synthetic catalysts, speed up the hydrolysis reactions.
References
1. “Amide definition and meaning – Collins English Dictionary”. www.collinsdictionary.com. Retrieved 15 April 2018.
2. “amide”. The American Heritage Dictionary of the English Language (5th ed.). HarperCollins.
3. “amide – Definition of amide in English by Oxford Dictionaries”. Oxford Dictionaries – English. Archived from the original on 2 April 2015. Retrieved 15 April 2018.
4. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”) (1997). Online corrected version: (2006–) “amides”. doi:10.1351/goldbook.A00266
5. “Chapter 21: Amides and Imides”. Nomenclature of Organic Compounds. Advances in Chemistry. Vol. 126. Washington, American Chemical Society. 1974. pp. 166–173. doi:10.1021/ba-1974-0126.ch021. ISBN 9780841201910.
6. Boonen, Jente; Bronselaer, Antoon; Nielandt, Joachim; Veryser, Lieselotte; De Tré, Guy; De Spiegeleer, Bart (2012). “Alkamid database: Chemistry, occurrence and functionality of plant N-alkylamides” (PDF). Journal of Ethnopharmacology. 142 (3): 563–590. doi:10.1016/j.jep.2012.05.038. hdl:1854/LU-2133714. PMID 22659196. Archived (PDF) from the original on 9 October 2022.
7.Organic Chemistry IUPAC Nomenclature. Rules C-821. Amides http://www.acdlabs.com/iupac/nomenclature/79/r79_540.htm Archived 21 January 2011 at the Wayback Machine.
8. Kemnitz, Carl R.; Loewen, Mark J. (2007). “”Amide Resonance” Correlates with a Breadth of C−N Rotation Barriers”. Journal of the American Chemical Society. 129 (9): 2521–8. doi:10.1021/ja0663024. PMID 17295481.
9.Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1