Bassma Maytham Oleiw δ ASST. Maha Jassim Manshad
Department of chemistry, Collage of Education for Pure Science.
ASST.Saly Naser Abbas
Department of Biology, Collage of Education for Pure Science,
Abstract : Diabetes mellitus is a metabolic process that leads to hyperglycemia. It causes diabetes low levels of the diabetic hormone insulin, damage to beta cells in the pancreatic islets leads to Type 1. Genetics are thought to hold a significant position factor in the onset of metabolic syndrome type 1, but how they interact around in identical twins is only about 40 % Bacteria are among the Environmental factors involved in this disease, especially type 1 diabetes, as previous studies have proven the association of about 14 bacterial strains as type 1 diabetes unfolds in animal as well as human models. Experimental animal studies and in vitro research show that different viruses are obviously capable of modulating T1D progression through various mechanisms, including direct beta cell activation, T cell activation activation, Molecular mimicry and the disappearance of regulatory T cells Information in rodent and within vitro pathogenesis of human T1D Potential for viral infection Our attitude toward the application has improved. Alterations Because the development of type 2 diabetes is significantly influenced by mutations in particular genes, such as those linked to insulin sensitivity. In relation to environmental variables diet and lifestyle habits have a significant impact on the disease’s start, also, Infections are associated with the onset of these metabolic factors, but the overall relationship between these factors is not yet established no well It does, and this knowledge may provide guidelines for dealing with bacterial infections in common diabetics
Introduction
Type 1 diabetes contributes to 5-10% of diabetes cases globally; The prevalence ranges from 0.7 in Shanghai per 100,000, a Chinese city30% of 100 thousand individuals in Finland. However, the frequency of this condition in children has risen significantly worldwide. Adding the type 1 diabetes cases of seventeen individuals resulted in an annual increase of 3.0% [1]. The T cells are thought to trigger an autoimmune response that attacks the beta cells in the organ known as the pancreas that produce insulin, leading to a condition known as type 1 diabetes All diabetics have type 2 diabetes, commonly referred to as type 2 diabetes. compared to T1D, classified as autoimmune, T2D is brought on by insulin resistance and an insufficient number of beta cells in the the spleen which, pursuant to a few study findings, is able to compensate for the loss of pancreatic beta cells. Before clinical indications such as weight loss, ketosis, and polyuria appear in type 1 diabetes, approximately 60–80% of the β-cells—specialized endocrine cells contained beneath the pancreatic islets of Langerhans—that manufacture and release insulin and amylin needs to be annihilated [2]. Other islet cells are usually spared when the pancreatic peptides, glucagon, and somatostatin are secreted. immune system strikes beta cells. Histologically, immunological barriers infiltration (insulitis) in type 1 diabetics occurs in the pancreatic islets, which include an elevated amount of CD8 + T cells and mononuclear cells [3].Due to a decline in β cell is significant, there was very little endogenous insulin manufacturing, which ends up in increased blood glucose in people with type 1. Introduction to the Edmonton Program, which relies on exogenous insulin to maintain normal blood sugar values [4] although this treatment is still limited to moderately diabetic patients or low blood sugar problems. Poor glycemic control in T1D patients improves their potential long-term risk of developing diabetic sequelae, which are categorized further as macrovascular (cardiovascular) conditions and microvascular (nephropathy, retinopathy, and neuropathy). There is not much dispute that a patient’s genetic background and environmental factors—like exercise and diet—have a direct effect on the emergence of type 2 diabetes.[5] Emerging evidence suggests that specific environmental factors may be involved in the risk of developing type 2 diabetes, particularly the accumulation of gut-dwelling microorganisms , known as humans more than half a dozen viruses are linked to diabetes type 2. (Table1 ) . These Viruses comprise virus coxsackie B [6,7] virus rubella [8,9] Mumps fever [10,11] [12,13] a virus called Epstein-Barr virus The zoster malware Varicella It has been reported that rotavirus and retrovirus exist. Nine viruses or more. Type 1 diabetes is a condition in development in rats (Table 2). Mengo, reovirus, retrovirus, encephalomyocarditis (EMC) virus is apparent and Coxsackievirus B, specially B4, foot-and-mouth disease malware in mice and primates that are not humans, porcine origin and bovine viruses, diarrhea, and mucositis virus have all been observed in mice; in addition to causing diabetes, Kelham rats treated with virus (KRV) has been identified among calves and rats. likewise There is a certain amount of proof. from laboratories that viruses such the murine hepatitis virus and the lymphocytic choroidal meningitis virus (LCMV) can spontaneously protect diabetic animals from developing the autoimmune disease T1D [14,15] regardless of the importance of the subject. This field has yet to be adequately investigated, and the information
available.
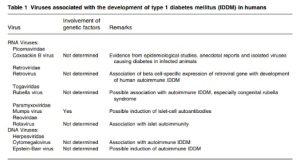

2. Viruses developed for diabetes
Viruses often use the cellular machinery of the host for their own benefit. As a secondary consequence, the metabolism of infected cells is reprogrammed to increase nutrient uptake to help the bacteria replicate. Interestingly, energy metabolism regulation is involved in the onset of diabetes [16] Some bacteria have a tropism for intestinal beta cells, whereas others induce specific beta cell immunity Certain bacterial strains can cause death of beta cells due to events cytopathic [17] Overproduction of cytokines that cause inflammation when infected Additionally, throughout the evolution of diabetes helps therefore metabolic reprogramming, of pancreatic cells Infections were the mechanisms by which viruses can cause diabetes
2.1 Viruses that development type 1 diabetes
2.1.1 Type 1 Diabetes with Viral Encephalomyelitis Pertaining to the Picornaviridae family, encephalomyocarditis (EMC) viruses are tiny, single-stranded RNA viruses with a genome size of about 7.8 kb, just like enteroviruses. Research on rodents clearly shows that EMC chromosome strains induce T1D in such animals, but the lack of evidence linking the EMC gene to the biology of T1D in humans [18].EMC variant M virus (EMC-M) diabetes in genetically susceptible mouse strains. Strip purification of the EMC-M virus that causes Similar symptoms [19] resulted in the diabetic EMC-D and diabetic EMC-B infections being totally distinct as two strong strains. More than 90% of afflicted animals develop diabetes as a result of the EMC-D virus. but the EMC-B becoming infected in mice nibbling infection not a cause of diabetes [20] Anti-T-cell antibody treatment of these mice did not prevent the growth of diabetes, indicating that the EMC-D virus and intestinal β-cells destroyed in SJL/J mice are EMC-D-infected mice rather than T-cell-mediated. Anti-macrophage immunoglobulin therapy gave rise to significantly lower diabetes.
2.1.2 Retrovirus infection and T1D
Two recent-onset T1D patients had pancreas lesions with A human endogenous retrovirus known as the mouse mammary tumor virus (MMTV) (IDDMK1, 222) suspected of it, but not non-diabetic controls. Cell injury [21] Nevertheless, evidence from additional research indicates that (IDDMK1, 222) does not accelerate the emergence of T1D, suggesting that it is unlikely that (IDDMK1, 222) playing a significant part in the physiological causes of T1D.
2.1.3 Kilham Rat Virus
The parvovirus family includes the Kelham murine virus (KRV). Parvoviruses make up of a linear DNA molecule and three proteins, and their sizes range from 18 to 26 nanometers. Parvoviruses spread to the bone marrow, lymph nodes, gut, and growing fetus because they replicate in the nucleus of proliferating cells.[22] Mixing the genome with infected cells Rather than directly activating beta cells, KRV has been found to cause diabetes in immune BB (DR-BB) mice by means of producing an autoimmune reaction against them. [23] Like NOD mice, diabetes-prone (DP)-BB mice commonly acquire spontaneous diabetes that resembles human T1D.
2.1.4 Mucosal disease caused by viral diarrhea in cows
Viruses The virus that causes bovine viral diarrheal mucosal disease (BVD-MD) is frequently observed in animals like cattle and is one of the members of the pestivirus family of the genus Flavivirus. Cattle with diabetes type 1 has been connected to the BVD-MD virus, but not animals with any other kind of the illness. It’s unclear that [23] this is because different viral strains exist or because hosts differ genetically. In a recent study, rats with T1D found were infected with BVD-MD demonstrated that the pancreas had BVD-MD genes, but not the islet cells. This suggests that BVD-MD has no direct impact on T1D islet cells linked to BVD-MD [24]. In order to determine whether BVD-MD indeed triggers a response in the immune system that results in
2.2 Viruses that development type 2 diabetes
2.2.1 Hepatitis C
Type 2 diabetes and insulin resistance are linked to chronic hepatitis C (CHC), yet the degree of insulin function loss, the target pathways implicated, and the significance of viruses themselves in T2DM have not been defined ) and high prevalence have been reported IR and people with type 2 diabetes with HCV have been linked to a number of different pathways. These pathways could involve the virus’s immediate effects on insulin signaling or its indirect influence via its generation of inflammatory cytokines notably tumor necrosis factor alpha (TNF-α). He has been picking skills up. Still, investigation has turned up an extended group of liver-derived proteins that have been demonstrated to impact lipid and glucose metabolism in the liver, fat tissue, and skeletal muscle. Major hepatokines governing human metabolism, especially HS-glycoprotein (also known as fetuin A)-have been identified as risk factors for type 2 diabetes (T2DM) based on their regulation of IR and human α2. It was discovered that this hepatokine naturally inhibits the insulin receptor’s tyrosine kinase in the skeletal muscle and liver. Furthermore, a high-fat meal both encouraged and hindered weight gain in mice mutant in the gene demonstrating gut A [25].
2.2.2 Cytomegalovirus
Within the host myeloid lineage, hematopoietic progenitor cells that are CD34+, human cytomegalovirus (CMV) genes are retained as extrachromosomal plasmids after an initial Asymptomatic or self-limited infection caused by lysis between infancy and adolescence. [26,27, 28]. UL81-1, which includes Dhiksactiva, cryptic CMV av Latency-associated unknown nuclear antigen (LUNA), differs from other Herpesviridae family viruses in that it can display several signatures, including antisense transcripts and latency-associated isoform. Despite the complexity of Adipocytokines, receptors, and genetic pathways are involved in DM, or non-insulin dependent diabetes mellitus, is a substitute term for type 2 diabetes mellitus (T2DM). still a range of functionally related compounds and the immune system are also entailed… With over a variety of micro- and macrovascular problems, type 2 diabetes (T2DM) appears to be essentially a chronic low metabolic syndrome [29, 30, 31, 32, 33, 34, 35] In situ hybridization and reverse transcription polymerase chain reaction (PCR) were used by Lohr and Oldstone [36] to identify immediate and late CMV genes in intestinal tissue from T2DM patients. Viperin (associated with endoplasmic reticulum; also known as interferon-inducible viral inhibitor protein), which is caused by CMV directly has been shown in recent studies to interact using the mitochondrial inhibitor CMV (vMIA) protein of apoptosis to influence lipid and glucose metabolism [37–38]. to supply They are able to
2.2.3 Severe acute respiratory syndrome coronaviru
The new coronavirus disease 2019 (COVID-19) coronavirus 2 (SARS-CoV-2) caused by severe respiratory illness poses a novel public health issue that has a substantial effect on global rates of morbidity and death [39,40]. There are reports of these. Release of cytokines as a result of acute respiratory illness Because coronavirus 2 infection alters glucose homeostasis, it may hasten the start of metabolic alterations[41] The current study’s two recent publications in Cell Metabolism offer more proof of the connection between hyperglycemia and SARS-CoV-2 infection [42, 43]. Reiterer et al. discovered that in 3854 hospitalized COVID-19 patients, hyperglycemia (generally defined as blood glucose >170 mg/dL) had a poor prognostic significance. included in patients ages 15.6–, 9.8–, and 16–12. linked to a 3.3-fold higher risk of mortality, ARDS, and intubation. Currently, there is little evidence that SARS-CoV-2 directly or indirectly affects beta cell function [44,45,46,47,48] Theoretically, The endocrine pancreas could act as a point of acquisition for SARS-CoV-2; in fact, mRNA levels of the enzyme angiotensin-converting enzyme 2 (ACE2), a major SARS-CoV-2 receptor, are present in the exocrine and endocrine pancreas [49,50] as well as in immunobone chemistry and in situ hybridization studies found that such high We detected SARS-CoV-1-related antigen in the intestinal tracts of SARS-CoV-1-deading patients. [51, 52, 53, 54] It’s interesting to remember who SARS-CoV-2 also causes a cytokine storm, an immunological irritable reaction characterized by a large-scale synthesis of cytokines that leads to systemic proinflammation.
2.2.4 Human immunodeficiency virus (HIV)
Individuals with HIV who are undergoing antiretroviral therapy (ART) may have anomalies related to their metabolism. Insulin resistance is reported by Bergersen et al. among HIV-negative people who are not receiving antiretroviral therapy (ART), ,[55,56] and the HIV viral load is a valid predictor of metabolic severity, as shown by a new study. Extra effort[57,58,59] During the maturation stage in The widely aid components Nef and Vpr in vitro are linked to changes in lipid synthesis and storage,[60] lipogenesis, and insulin resistance [61,62,63].In reaction to hyperglycemia, pancreatic β-cells release insulin, which activates muscles and prevents adipose tissue from holding onto sugar and the liver from giving the blood via glucose. When the liver permits glucose to be excreted and glucose absorption is not appropriately stimulated, insulin resistance develops. Type 2 diabetes eventually results from persistent diabetes. The prevalence Potential problems with glucose metabolism in people living with HIV is thought to range from 2% to 25% [64], with a 2.2-fold increased risk of developing type 2 diabetes [65] but in in a previous report on insulin withdrawal, . described the sensitivity of a subgroup of HIV-infected men f progression rates [66] . Although HIV is a disease.
3. Conclusion
Although viruses can be linked to the onset of T1DM and T2DM, genetic factors and lifestyle choices remain the primary predispositions for the development of these diseases; nonetheless, the precise contribution of virus infection to global DM incidence remains uncertain. Data released recently, for instance, shows that both hospitalized and non-hospitalized individuals with COVID-19 had a higher incidence of diabetes. Substantial extended observational research launched subsequent to viral epidemics are necessary to quantify the direct effect of viruses on diabetes mellitus. Though a number of viral pathways have already been related to diabetes mellitus, further research is necessary in order to fully understand the entire picture. considering that a pandemic virus linked to these diseases threatens millions of people with DM, more research is necessary to fully comprehend the relationship underlying viral illness and DM.
References
1-Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia 1999; 42: 1395–1403.
2-Greiner DL, Rossini AA, Mordes JP. Translating data from animal models into methods for preventing human autoimmune diabetes mellitus: caveat emptor and primum non nocere. Clin Immunol 2001; 100: 134–143.
3-Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest 2001; 108: 1247–1252.
4-Q. Yang, A. Vijayakumar, B.B. Kahn Metabolites as regulators of insulin sensitivity and metabolism Nat. Rev. Mol. Cell Biol., 19 (2018), pp. 654-672
5- Z. Qi, H. Hu, Z. Wang, G. Wang, Y. Li, X. Zhao, Y. Feng, X. Huo, J. Sun, Q. Feng, Y. Liu, N. Wang, C. Guo, Y. Li, R. Wang, J. Hu Antibodies against H1N1 influenza virus cross-react with α-cells of pancreatic islets J. Diabetes Invest., 9 (2018), pp. 265-
6-Shapiro AMJ, Ryan EA, Lakey JRT, et al. Using an immunosuppressive regimen free of glucocorticoids, seven patients with type 1 diabetes mellitus underwent islet transplantation. 2000; N Engl J Med 343: 230–238
7-The difficulty of type 1 diabetes mellitus, Eisenelin L, Schwartz HJ, Rutledge JC. ILAR J 2004; 45: 231–236.
8-Kominek HI, Yoon JW. The pathophysiology of diabetes mellitus involves the involvement of Coxsackie B viruses. Rose NR, Friedman H, eds., Microorganisms and Autoimmune Diseases. 129–158 in Plenum Press, New York, 1996
9-Gladisch R, Hoffmann W, Waldherr R. Myocarditis and insulitis following Coxsackie virus infection. Z Kardiol 1976: 65; 837-849
10-Jensen A, Rosenberg H, Notkins AL. Virus-induced diabetes mellitus XVII: pancreatic islet cell damage in children with fatal viral infections. Lancet 1980: 2; 354-358
11-Fedun B, Cooper LZ, Witt ME, Franklin BH, Roman SH, Rubenstein P, McEvoy RC, Ginsberg-Fellner F. Relationships between type 1 insulin-dependent diabetic mellitus and congenital rubella. Singh B, Rajotte RV, Molnar GD, Jaworski MA, eds., The Immunology of Diabetes Mellitus. Amsterdam, Elsevier, 1986; 279–286
12- Ginsberg-Fellner F, Witt ME, Yagihaski S. Congenital rubellasyndrome as a model for type 1 (insulin-dependent) diabetes mellitus: increased prevalence of islet cell surface antibodies. Diabetologia1984: 27; 87-89.
13-Gamble DR. Relation of antecedent illness to development of diabetes in children. Br Med J 1980: 2; 99-101.
14-Otten A, Willems W, and Helmke K. Islet cell antibodies in pediatric mumps patients (n = 14). 1980’s Lancet 2: 211-212.
15-Congenital cytomegalovirus infection with diabetes, Ward KP, Galloway WH, Auchterlonie IA. Lancet 1979: 1; 497.
16-50Ward KP, Galloway WH, Auchterlonie IA. Congenital cytomegalo-virus infection and diabetes. Lancet 1979; 1: 497.
17-Craighead JE, McLane MF. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science 1968; 162: 913–914.
18-Yoon JW, McClintock PR, Onodera T, et al. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med 1980; 152: 878–892.
19-Conrad B, Weissmahr RN, Boni J, et al. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell 1997; 90: 303–313.
20-Kim A, Jun HS, Wong L, et al. Human endogenous retrovirus with a high genomic sequence homology with IDDMK(1,2)22 is not specific for type I (insulin-dependent) diabetic patients but ubiquitous. Diabetologia 1999; 42: 413–418.
21-Kersh GJ, Allen PM. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J Exp Med 1996; 184: 1259–1268.
22-Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 2000; 49: 1319–1324.
23-Tajima M, Yazawa T, Hagiwara K, Kurosawa T, Takahashi K. Diabetes mellitus in cattle infected with bovine viral diarrhea mucosal disease virus. J Vet Med A 1992: 39; 616-620
24-Tajima M, Yuasa M, Kawanabe M, Taniyama H, Yamato O, Maede Y. Possible causes of diabetes mellitus in cattle infected with bovine diarrhoea virus. Zentralbl Veterinarmed [B] 1999: 46; 207-215.
25-Sahar A. Ali, Walaa M.H. Nassif, Dalia H.A. Abdelaziz Alterations in serum levels of fetuin A and selenoprotein P in chronic hepatitis C patients with concomitant type 2 diabetes: A case-control study September 2016: 465-470
26- Kumar A, Herbein G. Epigenetic regulation of human cytomegalovirus latency: an update. Epigenomics 2014;6:533-46.
27-Poole E, Sinclair J. Sleepless latency of human cytomegalovirus. Med Microbiol Immunol 2015;204:421-9.
28-Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20:202-13.
29- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141-50.
30- Grossmann V, Schmitt VH, Zeller T, Panova-Noeva M, Schulz A, Laubert-Reh D, Juenger C, Schnabel RB, Abt TG, Laskowski R, Wiltink J, Schulz E, Blankenberg S, Lackner KJ, Munzel T, Wild PS. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care2015;38:1356-64.
31-Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017;60: 1620-9.
32-Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother 2018;101:287-92.
33- Szabo M, Mate B, Csep K, Benedek T. Genetic approaches to the study of gene variants and their impact on the pathophysiology of type 2 diabetes. Biochem Genet 2018;56:22-55.
34-Kerru N, Singh-Pillay A, Awolade P, Singh P. Current anti-diabetic agents and their molecular targets: a review. Eur J Med Chem 2018;152:436-88.
35-Ashoori MR, Rahmati-Yamchi M, Ostadrahimi A, Fekri Aval S, Zarghami N. MicroRNAs and adipocytokines: promising biomarkers for pharmacological targets in diabetes mellitus and its complications. Biomed Pharmacother 2017;93:1326-36.
36-Lohr JM, Oldstone MB. Detection of cytomegalovirus nucleic acid sequences in pancreas in type 2 diabetes. Lancet 1990;336: 644-8.
37-Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science 2011;332:1093-7.
38-Seo JY, Cresswell P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog 2013;9: e1003497.
39-D. Tsilingiris, N.G. Vallianou, I. Karampela, M. Dalamaga Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story Metabolism open, 11 (2021), p. 100101,
40-N.G. Vallianou, D. Tsilingiris, G.S. Christodoulatos, I. Karampela, M. Dalamaga Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic Metabol Open, 10 (2021), p. 100096,
41-L. Montefusco, M. ben Nasr, F. D’Addio, C. Loretelli, A. Rossi, I. Pastore, G. Daniele, A. Abdelsalam, A. Maestroni, M. Dell’Acqua, E. Ippolito, E. Assi, V. Usuelli, A.J. Seelam, R.M. Fiorina, E. Chebat, P. Morpurgo, M.E. Lunati, A.M. Bolla, G. Finzi, R. Abdi, J.v. Bonventre, S. Rusconi, A. Riva, D. Corradi, P. Santus, M. Nebuloni, F. Folli, G.V. Zuccotti, M. Galli, P. Fiorina Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection Nat. Metab., 3 (2021), pp. 774-785
42-M. Reiterer, M. Rajan, N. Gomez-Banoy, J.D. Lau, L.G. Gomez-Escobar, L. Ma, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2 Cell Metabol, 33 (2021), pp. 2174-2188,
43-M. Zickler, S. Stanelle-Bertram, S. Ehret, F. Heinrich, P. Lange, B. Schaumburg, et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans Cell Metabol (2021),
44-Yang, J. K., Lin, S. S., Ji, X. J. & Guo, L. M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 47, 193–199 (2010).
45-Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8, 782–792 (2020).
46-Steenblock, C., et al. Beta cells from patients with COVID-19 and from isolated human islets exhibit ACE2, DPP4 and TMPRSS2 expression, viral infiltration and necroptotic cell death. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-88524/v1 (2020).
47-Kusmartseva, I. et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 32, 1041–1051 (2020).
48-Yang, L. et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 27, 125–136 (2020).
49-Liu, F. et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 18, 2128–2130 (2020).
50-Ding, Y. et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 203, 622–630 (2004).
51-Bornstein, S. R. et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 8, 546–550 (2020).
52- Dave, G. S. & Kalia, K. Hyperglycemia induced oxidative stress in type-1 and type-2 diabetic patients with and without nephropathy. Cell Mol. Biol. 53, 68–78 (2007).
53-de Carvalho Vidigal, F., Guedes Cocate, P., Goncalves Pereira, L. & de Cassia Goncalves Alfenas, R. The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutr. Hosp. 27, 1391–1398 (2012).
54-Fabbri, A. et al. Stress hyperglycemia and mortality in subjects with diabetes and sepsis. Crit. Care Explor. 2, e0152 (2020).
55-Niewczas, M. A. et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 25, 805–813 (2019).
56-Folli, F. et al. Proteomics reveals novel oxidative and glycolytic mechanisms in type 1 diabetic patients’ skin which are normalized by kidney–pancreas transplantation. PLoS ONE 5, e9923 (2010).
57-Bassi, R. & Fiorina, P. Impact of islet transplantation on diabetes complications and quality of life. Curr. Diab. Rep. 11, 355–363 (2011).
58-La Rocca, E. et al. Patient survival and cardiovascular events after kidney–pancreas transplantation: comparison with kidney transplantation alone in uremic IDDM patients. Cell Transplant. 9, 929–932 (2000).
59-Rasheed S, Yan JS, Lau A, Chan AS. HIV replication enhances production of free fatty acids, low density lipoproteins and many key proteins involved in lipid metabolism: a proteomics study, PLoS One, 2008, vol. 3 pg. e3003
60-Squillace N, Zona S, Stentarelli C, et al. Detectable HIV viral load is associated with metabolic syndrome, J Acquir Immune Defic Syndr, 2009, vol. 52 (pg. 459-64)
61-Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients, Diabetes, 2005, vol. 54 (pg. 23-31)
62-van ‘t Wout AB, Swain JV, Schindler M, et al. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells, J Virol, 2005, vol. 79 (pg. 10053-8)
63-Otake K, Omoto S, Yamamoto T, et al. HIV-1 Nef protein in the nucleus influences adipogenesis as well as viral transcription through the peroxisome proliferator-activated receptors, AIDS, 2004, vol. 18 (pg. 189-98)
64-Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy, J Acquir Immune Defic Syndr, 2009, vol. 50 (pg. 499-505)
65-Li X, Brown TT, Cole SR, et al. Antiretroviral therapy and the multicenter AIDS cohort study’s prevalence and incidence of diabetes mellitus, Arch Intern Med, vol. 165, 2005, pp. 1179–84
66-Hommes MJ, Sauerwein HP, Romijn JA, Endert E, and Eeftinck Schattenkerk JK were among the 66. Human immunodeficiency virus-infected men’s insulin sensitivity and insulin clearance, Metabolism, 1991, vol. 40, pp. 651-6





























































